Tislelizumab
Tislelizumab is an antibody pharmaceutical. It is currently being investigated in clinical studies. The pharmaceutical is active against programmed cell death protein 1.
Download report
Favorite
Commercial
Therapeutic Areas
No data
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
New Drug Application (NDA)
New Drug Application (NDA)
Abbreviated New Drug Application (ANDA)
Abbreviated New Drug Application (ANDA)
No data
Labels
FDA
EMA
No data
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
HCPCS
No data
Clinical
Clinical Trials
311 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
No data
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Non-small-cell lung carcinoma | D002289 | 7 | 17 | 9 | — | 1 | 32 | ||
| Neoplasms | D009369 | C80 | 16 | 12 | 1 | — | — | 21 | |
| Liver neoplasms | D008113 | EFO_1001513 | C22.0 | 3 | 13 | 1 | — | 5 | 21 |
| Esophageal squamous cell carcinoma | D000077277 | 1 | 11 | 4 | — | 1 | 15 | ||
| Stomach neoplasms | D013274 | EFO_0003897 | C16 | 5 | 11 | 2 | — | — | 14 |
| Hepatocellular carcinoma | D006528 | C22.0 | 1 | 8 | 1 | — | — | 10 | |
| Small cell lung carcinoma | D055752 | 2 | 6 | 1 | — | 1 | 8 | ||
| Squamous cell carcinoma of head and neck | D000077195 | 4 | 3 | 1 | — | — | 8 | ||
| Hodgkin disease | D006689 | C81 | — | 6 | 1 | — | — | 7 | |
| Nasopharyngeal neoplasms | D009303 | 1 | 3 | 2 | — | — | 6 |
Show 5 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Colorectal neoplasms | D015179 | 6 | 18 | — | — | 1 | 21 | ||
| Immunotherapy | D007167 | — | 8 | — | — | — | 8 | ||
| Cholangiocarcinoma | D018281 | C22.1 | 1 | 7 | — | — | — | 7 | |
| Melanoma | D008545 | 3 | 6 | — | — | — | 7 | ||
| Uterine cervical neoplasms | D002583 | 1 | 6 | — | — | — | 7 | ||
| Neoadjuvant therapy | D020360 | — | 6 | — | — | — | 6 | ||
| Triple negative breast neoplasms | D064726 | 2 | 6 | — | — | — | 6 | ||
| Urinary bladder neoplasms | D001749 | C67 | 2 | 6 | — | — | — | 6 | |
| Rectal neoplasms | D012004 | — | 4 | — | — | 1 | 5 | ||
| Pancreatic neoplasms | D010190 | EFO_0003860 | C25 | 1 | 5 | — | — | — | 5 |
Show 47 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Mesothelioma | D008654 | C45 | 2 | — | — | — | — | 2 | |
| Leukemia | D007938 | C95 | 1 | — | — | — | — | 1 | |
| Adenoid cystic carcinoma | D003528 | 1 | — | — | — | — | 1 | ||
| Endometrial neoplasms | D016889 | EFO_0004230 | 1 | — | — | — | — | 1 | |
| Hereditary nonpolyposis colorectal neoplasms | D003123 | EFO_0007354 | 1 | — | — | — | — | 1 | |
| Acinar cell carcinoma | D018267 | 1 | — | — | — | — | 1 | ||
| Pancreatic ductal carcinoma | D021441 | 1 | — | — | — | — | 1 | ||
| Anus neoplasms | D001005 | EFO_0003835 | C21 | 1 | — | — | — | — | 1 |
| Ovarian epithelial carcinoma | D000077216 | 1 | — | — | — | — | 1 | ||
| Thymus neoplasms | D013953 | C37 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Liver diseases | D008107 | EFO_0001421 | K70-K77 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | TISLELIZUMAB |
| INN | tislelizumab |
| Description | Tislelizumab (BGB-A317) is a humanized monoclonal antibody directed against PD-1. It prevents PD-1 from binding to the ligands PD-L1 and PD-L2 (hence it is a checkpoint inhibitor). It is being investigated as a treatment for advanced solid tumors.
|
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 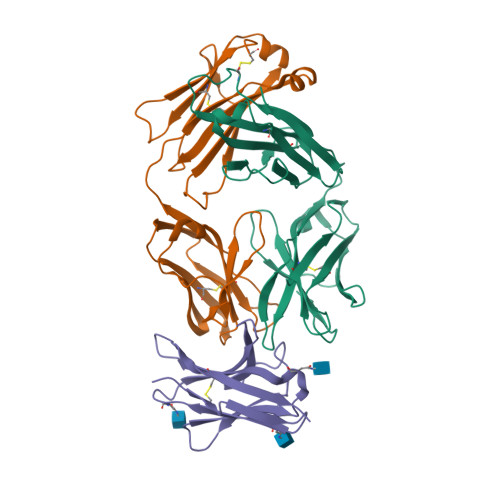 |
| Structure (InChI/SMILES or Protein Sequence) | >7CGW:A,H|Heavy chain of tislelizumab Fab
QVQLQESGPGLVKPSETLSLTCTVSGFSLTSYGVHWIRQPPGKGLEWIGVIYADGSTNYNPSLKSRVTISKDTSKNQVSL
KLSSVTAADTAVYYCARAYGNYWYIDVWGQGTTVTVSSASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWN
SGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTKTYTCNVDHKPSNTKVDKRVESKYGPHHHHHH
>7CGW:B,L|Light chain of tislelizumab Fab
DIVMTQSPDSLAVSLGERATINCKSSESVSNDVAWYQQKPGQPPKLLINYAFHRFTGVPDRFSGSGYGTDFTLTISSLQA
EDVAVYYCHQAYSSPYTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQ
ESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Identifiers
| PDB | 7BXA, 7CGW |
| CAS-ID | 1858168-59-8 |
| RxCUI | — |
| ChEMBL ID | CHEMBL4297840 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB14922 |
| UNII ID | 0KVO411B3N (ChemIDplus, GSRS) |
Target
Agency Approved
PDCD1
PDCD1
Alternate
No data
Variants
Clinical Variant
No data
Financial
Tislelizumab - Beigene
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 1,476 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
66 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
