Remicade(infliximab)
Avsola, Inflectra, Remicade, Renflexis (infliximab) is an antibody pharmaceutical. Infliximab was first approved as Remicade on 1998-08-24. It is used to treat ankylosing spondylitis, crohn disease, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis in the USA. It has been approved in Europe to treat ankylosing spondylitis, crohn disease, psoriasis, psoriatic arthritis, and rheumatoid arthritis amongst others. The pharmaceutical is active against tumor necrosis factor.
Download report
Favorite
COVID-19
Top 200 Pharmaceuticals by Retail Sales
Commercial
Trade Name
FDA
EMA
Avsola, Inflectra, Remicade, Renflexis (discontinued: Ixifi)
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Infliximab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Remicade | infliximab | Johnson & Johnson | N-103772 RX | 1998-08-24 | 1 products |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| ankylosing spondylitis | EFO_0003898 | D013167 | M45 |
| crohn disease | EFO_0000384 | D003424 | K50 |
| psoriatic arthritis | EFO_0003778 | D015535 | L40.5 |
| rheumatoid arthritis | EFO_0000685 | D001172 | M06.9 |
| ulcerative colitis | EFO_0000729 | D003093 | K51 |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
infliximab, Remicade, Janssen Biotech, Inc. | |||
| 2118-09-23 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| EJ | Subsequent claims for a defined course of therapy, e.g., epo, sodium hyaluronate, infliximab |
| J1745 | Injection, infliximab, excludes biosimilar, 10 mg |
| Q5103 | Injection, infliximab-dyyb, biosimilar, (inflectra), 10 mg |
| Q5104 | Injection, infliximab-abda, biosimilar, (renflexis), 10 mg |
| Q5109 | Injection, infliximab-qbtx, biosimilar, (ixifi), 10 mg |
| Q5121 | Injection, infliximab-axxq, biosimilar, (avsola), 10 mg |
| S9359 | Home infusion therapy, anti-tumor necrosis factor intravenous therapy; (e.g., infliximab); administrative services, professional pharmacy services, care coordination, and all necessary supplies and equipment (drugs and nursing visits coded separately), per diem |
Clinical
Clinical Trials
370 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Crohn disease | D003424 | EFO_0000384 | K50 | 1 | 3 | 18 | 23 | 38 | 81 |
| Rheumatoid arthritis | D001172 | EFO_0000685 | M06.9 | 2 | 4 | 24 | 22 | 26 | 78 |
| Ulcerative colitis | D003093 | EFO_0000729 | K51 | 2 | — | 10 | 17 | 17 | 46 |
| Inflammatory bowel diseases | D015212 | EFO_0003767 | 2 | 1 | 1 | 8 | 23 | 34 | |
| Ankylosing spondylitis | D013167 | EFO_0003898 | M45 | 3 | — | 5 | 11 | 11 | 30 |
| Psoriasis | D011565 | EFO_0000676 | L40 | — | 2 | 11 | 4 | 12 | 29 |
| Psoriatic arthritis | D015535 | EFO_0003778 | L40.5 | — | — | 4 | 5 | 6 | 15 |
| Covid-19 | D000086382 | U07.1 | — | 2 | 2 | 1 | 1 | 6 | |
| Graft vs host disease | D006086 | D89.81 | 1 | 2 | 1 | 1 | — | 5 | |
| Uveitis | D014605 | EFO_1001231 | H20.9 | 1 | 1 | — | 1 | 2 | 4 |
Show 14 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Mucocutaneous lymph node syndrome | D009080 | EFO_0004246 | M30.3 | 1 | — | 5 | — | — | 6 |
| Behcet syndrome | D001528 | EFO_0003780 | M35.2 | 1 | 1 | 3 | — | — | 4 |
| Vasculitis | D014657 | EFO_0006803 | M31 | 1 | 2 | 1 | — | — | 3 |
| Lung neoplasms | D008175 | C34.90 | 1 | 2 | 1 | — | — | 3 | |
| Cachexia | D002100 | HP_0004326 | R64 | — | 2 | 1 | — | — | 3 |
| Juvenile arthritis | D001171 | EFO_0002609 | M08 | — | 1 | 2 | — | — | 3 |
| Fatigue | D005221 | HP_0012378 | R53.83 | — | 1 | 1 | — | — | 2 |
| Macular edema | D008269 | 1 | 1 | 1 | — | — | 2 | ||
| Diabetic retinopathy | D003930 | EFO_0003770 | 1 | — | 1 | — | — | 2 | |
| Chronic obstructive pulmonary disease | D029424 | EFO_0000341 | J44.9 | — | 1 | 1 | — | — | 2 |
Show 11 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Melanoma | D008545 | 2 | 4 | — | — | 1 | 6 | ||
| Colitis | D003092 | EFO_0003872 | K52.9 | 2 | 4 | — | — | 1 | 5 |
| Takayasu arteritis | D013625 | EFO_1001857 | M31.4 | — | 3 | — | — | 1 | 4 |
| Stevens-johnson syndrome | D013262 | EFO_0004276 | L51.1 | 2 | 3 | — | — | — | 3 |
| Macular degeneration | D008268 | EFO_0001365 | H35.30 | 1 | 1 | — | — | — | 2 |
| Non-small-cell lung carcinoma | D002289 | 2 | 2 | — | — | — | 2 | ||
| Reperfusion injury | D015427 | 1 | 1 | — | — | — | 2 | ||
| Hidradenitis suppurativa | D017497 | L73.2 | 1 | 1 | — | — | — | 2 | |
| Granulomatosis with polyangiitis | D014890 | EFO_0005297 | M31.3 | — | 1 | — | — | 1 | 2 |
| Microscopic polyangiitis | D055953 | EFO_1000784 | M31.7 | — | 1 | — | — | 1 | 2 |
Show 37 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers/patients | — | 3 | — | — | — | — | 3 | ||
| Scleritis | D015423 | HP_0100534 | H15.0 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Intestinal diseases | D007410 | HP_0002242 | K63.9 | — | — | — | — | 2 | 2 |
| Atopic dermatitis | D003876 | EFO_0000274 | L20 | — | — | — | — | 1 | 1 |
| Infections | D007239 | EFO_0000544 | — | — | — | — | 1 | 1 | |
| Cytokine release syndrome | D000080424 | D89.83 | — | — | — | — | 1 | 1 | |
| Kidney transplantation | D016030 | — | — | — | — | 1 | 1 | ||
| Rheumatic diseases | D012216 | M79.0 | — | — | — | — | 1 | 1 | |
| Gastrointestinal hemorrhage | D006471 | K92.2 | — | — | — | — | 1 | 1 | |
| Celiac disease | D002446 | EFO_0001060 | K90.0 | — | — | — | — | 1 | 1 |
| Ulcer | D014456 | MPATH_579 | — | — | — | — | 1 | 1 | |
| Pyoderma gangrenosum | D017511 | EFO_0006835 | L88 | — | — | — | — | 1 | 1 |
Show 3 more
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | INFLIXIMAB |
| INN | infliximab |
| Description | Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.
|
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 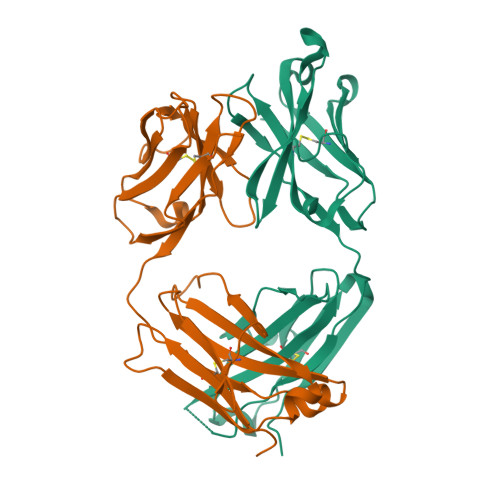 |
| Structure (InChI/SMILES or Protein Sequence) | >6UGS:H,A|Infliximab (Remicade) Fab Heavy Chain
EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMNWVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRFTISRDDSKSA
VYLQMTDLRTEDTGVYYCSRNYYGSTYDYWGQGTTLTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKT
>6UGS:L,B|Infliximab (Remicade) Fab Light Chain
DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHWYQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTDFTLSINTVES
EDIADYYCQQSHSWPFTFGSGTNLEVKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQ
ESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Target
Agency Approved
TNF
TNF
Organism
Homo sapiens
Gene name
TNF
Gene synonyms
TNFA, TNFSF2
NCBI Gene ID
Protein name
tumor necrosis factor
Protein synonyms
APC1 protein, Cachectin, TNF, macrophage-derived, TNF, monocyte-derived, TNF-a, TNF-alpha, tumor necrosis factor ligand 1F, Tumor necrosis factor ligand superfamily member 2, tumor necrosis factor-alpha, tumor necrotic factor alpha
Uniprot ID
Mouse ortholog
Tnf (21926)
tumor necrosis factor (P06804)
Alternate
No data
Variants
Clinical Variant
No data
Financial
Inflectra - Pfizer
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Flixabi - Biogen
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Renflexis - Organon
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Remicade - Johnson & Johnson
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Remicade - Merck Sharp & Dohme
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 38,864 documents
View more details
Safety
Black-box Warning
Black-box warning for: Avsola, Inflectra, Remicade, Renflexis
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
95 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
