Thrombin
Evicel, TachoSil, VeraSeal (thrombin) is an unknown pharmaceutical. Thrombin was first approved as Tachosil on 2004-06-08. It is used to treat hemorrhage in the USA. It has been approved in Europe to treat hemostasis and surgical hemostasis.
Download report
Favorite
Commercial
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
New Drug Application (NDA)
New Drug Application (NDA)
Abbreviated New Drug Application (ANDA)
Abbreviated New Drug Application (ANDA)
No data
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| tachosil | Biologic Licensing Application | 2022-11-02 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| hemorrhage | MP_0001914 | D006470 | R58 |
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
Clinical
Clinical Trials
238 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Hemorrhage | D006470 | MP_0001914 | R58 | — | 4 | 6 | 6 | 7 | 23 |
| Hemophilia a | D006467 | EFO_0007267 | D66 | — | 1 | 1 | 1 | 6 | 8 |
| Hemostasis | D006487 | 1 | 2 | — | 3 | 2 | 7 | ||
| Breast neoplasms | D001943 | EFO_0003869 | C50 | — | — | 2 | 1 | 3 | 6 |
| Surgical blood loss | D016063 | — | — | 1 | 1 | 3 | 5 | ||
| Anastomotic leak | D057868 | — | 1 | 1 | 2 | 1 | 5 | ||
| Intracranial hemorrhages | D020300 | EFO_0000551 | I62 | 1 | — | — | 2 | 1 | 4 |
| Atrial fibrillation | D001281 | EFO_0000275 | I48.0 | — | — | — | 1 | 2 | 3 |
| Lymphocele | D008210 | — | — | 2 | 1 | — | 3 | ||
| Uterine cervical neoplasms | D002583 | — | — | 1 | 2 | — | 3 |
Show 18 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Pterygium | D011625 | H11.0 | 2 | 1 | 2 | — | 5 | 9 | |
| Replacement arthroplasty knee | D019645 | — | — | 2 | — | 2 | 4 | ||
| Peripheral vascular diseases | D016491 | EFO_0003875 | I73.9 | — | — | 1 | — | 2 | 3 |
| Cerebrospinal fluid leak | D065634 | G96.0 | — | 1 | 2 | — | — | 3 | |
| Rectal fistula | D012003 | HP_0010447 | K60.3 | — | — | 3 | — | — | 3 |
| Wounds and injuries | D014947 | T14.8 | — | — | 2 | — | 1 | 3 | |
| Liver diseases | D008107 | EFO_0001421 | K70-K77 | — | — | 1 | — | 2 | 3 |
| Burns | D002056 | T30.0 | 1 | 2 | 1 | — | 1 | 3 | |
| Crohn disease | D003424 | EFO_0000384 | K50 | — | — | 1 | — | 1 | 2 |
| Cardiopulmonary bypass | D002315 | — | — | 1 | — | 1 | 2 |
Show 26 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Postoperative hemorrhage | D019106 | — | 2 | — | — | 1 | 3 | ||
| Seroma | D049291 | — | 1 | — | — | 1 | 2 | ||
| Chronic periodontitis | D055113 | EFO_0006343 | K05.3 | 2 | 1 | — | — | — | 2 |
| Rotator cuff injuries | D000070636 | M75.1 | — | 2 | — | — | — | 2 | |
| Infections | D007239 | EFO_0000544 | — | 1 | — | — | — | 1 | |
| Gallstones | D042882 | EFO_0004210 | — | 1 | — | — | — | 1 | |
| Pathologic processes | D010335 | — | 1 | — | — | — | 1 | ||
| Postoperative pain | D010149 | G89.18 | — | 1 | — | — | — | 1 | |
| Thromboembolism | D013923 | HP_0001907 | — | 1 | — | — | — | 1 | |
| Venus | D018536 | — | 1 | — | — | — | 1 |
Show 7 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Corneal ulcer | D003320 | H16.0 | 1 | — | — | — | 1 | 2 | |
| Healthy volunteers/patients | — | 2 | — | — | — | — | 2 | ||
| Intraabdominal infections | D059413 | 1 | — | — | — | — | 1 | ||
| Intestinal fistula | D007412 | K63.2 | 1 | — | — | — | — | 1 | |
| Periodontal diseases | D010510 | K05.6 | 1 | — | — | — | — | 1 | |
| Periodontal pocket | D010514 | EFO_1001393 | 1 | — | — | — | — | 1 | |
| Periodontal attachment loss | D017622 | 1 | — | — | — | — | 1 | ||
| Abdominal hernia | D046449 | K46 | 1 | — | — | — | — | 1 | |
| Ventral hernia | D006555 | EFO_1001866 | K43 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Covid-19 | D000086382 | U07.1 | — | — | — | — | 3 | 3 | |
| Acute coronary syndrome | D054058 | EFO_0005672 | — | — | — | — | 2 | 2 | |
| Inguinal hernia | D006552 | HP_0000023 | K40 | — | — | — | — | 2 | 2 |
| Blood coagulation disorders | D001778 | EFO_0009314 | D68.9 | — | — | — | — | 2 | 2 |
| Disseminated intravascular coagulation | D004211 | HP_0005521 | D65 | — | — | — | — | 2 | 2 |
| Cataract | D002386 | EFO_0001059 | H26.9 | — | — | — | — | 1 | 1 |
| Retinal detachment | D012163 | EFO_0005773 | H33.2 | — | — | — | — | 1 | 1 |
| Embolism and thrombosis | D016769 | — | — | — | — | 1 | 1 | ||
| Fibrosis | D005355 | — | — | — | — | 1 | 1 | ||
| Coronary disease | D003327 | — | — | — | — | 1 | 1 |
Show 33 more
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | THROMBIN |
| INN | thrombin |
| Description | Thrombin (EC 3.4.21.5, fibrinogenase, thrombase, thrombofort, topical, thrombin-C, tropostasin, activated blood-coagulation factor II, blood-coagulation factor IIa, factor IIa, E thrombin, beta-thrombin, gamma-thrombin) is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin (coagulation factor II) is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.
|
| Classification | Unknown |
| Drug class | — |
| Image (chem structure or protein) | 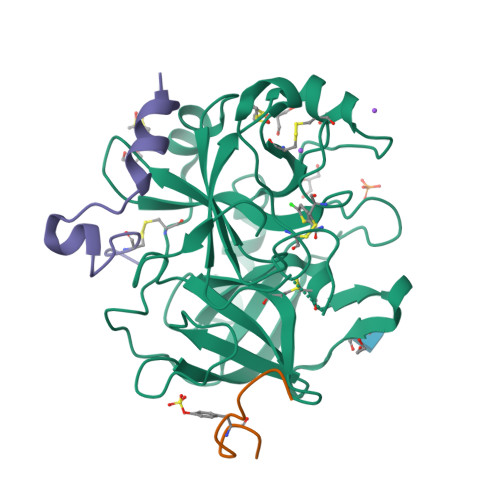 |
| Structure (InChI/SMILES or Protein Sequence) | >4UD9:H|Thrombin heavy chain
IVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTENDLLVRIGKHSRTRYERNIEKISM
LEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHPVCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVL
QVVNLPIVERPVCKDSTRIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKYGFY
THVFRLKKWIQKVIDQFGE
>4UD9:I|Hirudin-2
GDFEEIPEE(TYS)LQ
>4UD9:L|Prothrombin
EADCGLRPLFEKKSLEDKTERELLESYI |
Identifiers
Target
Agency Approved
No data
Alternate
No data
Variants
Clinical Variant
Identifier | Target mutation | Effect | Evaluation | Status |
|---|---|---|---|---|
| VCV000003520 | MTHFR, 665C>T, Ala222Val | drug response | 2021-10-21 | 2A |
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 103,592 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
2,919 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
