Dornase alfa
Pulmozyme (dornase alfa) is an enzyme pharmaceutical. Dornase alfa was first approved as Pulmozyme on 1993-12-30. It is used to treat bronchitis and cystic fibrosis in the USA.
Download report
Favorite
COVID-19
Commercial
Trade Name
FDA
EMA
Pulmozyme
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Dornase alfa
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Pulmozyme | dornase alfa | Genentech | N-103532 RX | 1993-12-30 | 1 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| pulmozyme | Biologic Licensing Application | 2020-10-21 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| bronchitis | — | D001991 | J40 |
| cystic fibrosis | EFO_0000390 | D003550 | E84 |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
dornase alfa, Pulmozyme, Genentech, Inc. | |||
| 2100-12-30 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J7639 | Dornase alfa, inhalation solution, fda-approved final product, non-compounded, administered through dme, unit dose form, per milligram |
Clinical
Clinical Trials
46 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cystic fibrosis | D003550 | EFO_0000390 | E84 | — | 3 | 4 | 5 | 2 | 14 |
| Pulmonary atelectasis | D001261 | J98.1 | — | — | — | 1 | 2 | 3 | |
| Pulmonary infarction | D054060 | EFO_1001408 | — | — | — | 1 | — | 1 | |
| Otitis media | D010033 | EFO_0004992 | H66.9 | — | — | — | 1 | — | 1 |
| Pneumonia | D011014 | EFO_0003106 | J18 | — | — | — | 1 | — | 1 |
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Covid-19 | D000086382 | U07.1 | 1 | 5 | 1 | — | 1 | 8 | |
| Respiratory distress syndrome | D012128 | EFO_1000637 | J80 | — | — | 2 | — | — | 2 |
| Sinusitis | D012852 | EFO_0007486 | J32 | — | 1 | 1 | — | — | 2 |
| Fibrosis | D005355 | — | — | 1 | — | — | 1 | ||
| Sars-cov-2 | D000086402 | — | — | 1 | — | — | 1 | ||
| Multiple trauma | D009104 | T07 | — | — | 1 | — | — | 1 | |
| Pleural empyema | D016724 | J86 | — | 1 | 1 | — | — | 1 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Ischemic stroke | D000083242 | — | 2 | — | — | — | 2 | ||
| Hypoxia | D000860 | R09.02 | — | 1 | — | — | — | 1 | |
| Dry eye syndromes | D015352 | H04.12 | 1 | 1 | — | — | — | 1 | |
| Respiratory tract infections | D012141 | J06.9 | — | 1 | — | — | — | 1 | |
| Asthma | D001249 | EFO_0000270 | J45 | — | 1 | — | — | — | 1 |
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers/patients | — | 1 | — | — | — | — | 1 | ||
| Malignant pleural effusion | D016066 | J91.0 | 1 | — | — | — | — | 1 | |
| Sjogren's syndrome | D012859 | EFO_0000699 | M35.0 | 1 | — | — | — | — | 1 |
| Cough | D003371 | HP_0012735 | R05 | 1 | — | — | — | — | 1 |
| Head and neck neoplasms | D006258 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Artificial respiration | D012121 | — | — | — | — | 2 | 2 | ||
| Respiratory insufficiency | D012131 | HP_0002093 | J96.9 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | DORNASE ALFA |
| INN | dornase alfa |
| Description | Deoxyribonuclease I precursor (DNase I) (Dornase alfa) |
| Classification | Enzyme |
| Drug class | enzymes |
| Image (chem structure or protein) | 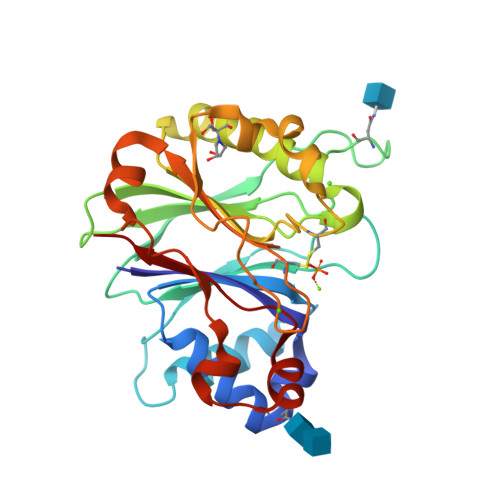 |
| Structure (InChI/SMILES or Protein Sequence) | — |
Identifiers
| PDB | 4AWN |
| CAS-ID | 9003-98-9 |
| RxCUI | 337623 |
| ChEMBL ID | CHEMBL1201431 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00003 |
| UNII ID | 953A26OA1Y (ChemIDplus, GSRS) |
Target
Agency Approved
No data
Alternate
No data
Variants
Clinical Variant
No data
Financial
Pulmozyme - Roche
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 1,595 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
163 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
