Erbitux(cetuximab)
Erbitux (cetuximab) is an antibody pharmaceutical. Cetuximab was first approved as Erbitux on 2004-02-12. It is used to treat squamous cell carcinoma in the USA. It has been approved in Europe to treat colorectal neoplasms and head and neck neoplasms. The pharmaceutical is active against epidermal growth factor receptor.
Download report
Favorite
Commercial
Trade Name
FDA
EMA
Erbitux
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Cetuximab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Erbitux | cetuximab | Eli Lilly | N-125084 RX | 2004-02-12 | 2 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| erbitux | Biologic Licensing Application | 2021-02-22 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| squamous cell carcinoma | — | D002294 | — |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
cetuximab, Erbitux, Eli Lilly and Company | |||
| 2113-03-01 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J9055 | Injection, cetuximab, 10 mg |
Clinical
Clinical Trials
831 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Colorectal neoplasms | D015179 | 70 | 180 | 44 | 3 | 17 | 278 | ||
| Head and neck neoplasms | D006258 | 35 | 79 | 14 | 2 | 7 | 125 | ||
| Neoplasms | D009369 | C80 | 56 | 35 | 2 | 1 | — | 77 | |
| Neoplasm metastasis | D009362 | EFO_0009708 | 3 | 8 | 2 | 2 | — | 13 | |
| Liver neoplasms | D008113 | EFO_1001513 | C22.0 | 3 | 4 | — | 1 | — | 7 |
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Squamous cell carcinoma of head and neck | D000077195 | 47 | 69 | 20 | — | 4 | 118 | ||
| Non-small-cell lung carcinoma | D002289 | 23 | 41 | 5 | — | — | 58 | ||
| Rectal neoplasms | D012004 | 14 | 22 | 1 | — | 2 | 34 | ||
| Colonic neoplasms | D003110 | C18 | 17 | 16 | 3 | — | 1 | 33 | |
| Squamous cell carcinoma | D002294 | 13 | 19 | 3 | — | 4 | 30 | ||
| Pancreatic neoplasms | D010190 | EFO_0003860 | C25 | 11 | 21 | 1 | — | 2 | 30 |
| Squamous cell neoplasms | D018307 | 9 | 23 | 2 | — | 2 | 27 | ||
| Esophageal neoplasms | D004938 | C15 | 6 | 21 | 3 | — | — | 24 | |
| Lung neoplasms | D008175 | C34.90 | 4 | 16 | 1 | — | — | 20 | |
| Adenocarcinoma | D000230 | 6 | 14 | 3 | — | — | 18 |
Show 10 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Breast neoplasms | D001943 | EFO_0003869 | C50 | 8 | 10 | — | — | — | 16 |
| Verrucous carcinoma | D018289 | 7 | 5 | — | — | 1 | 11 | ||
| Salivary gland neoplasms | D012468 | EFO_0003826 | D11 | 7 | 5 | — | — | — | 10 |
| Tongue neoplasms | D014062 | EFO_0003871 | C02.9 | 6 | 4 | — | — | 1 | 10 |
| Uterine cervical neoplasms | D002583 | 3 | 8 | — | — | — | 9 | ||
| Glioblastoma | D005909 | EFO_0000515 | 5 | 5 | — | — | — | 7 | |
| Renal cell carcinoma | D002292 | 5 | 6 | — | — | — | 7 | ||
| Ovarian neoplasms | D010051 | EFO_0003893 | C56 | 3 | 4 | — | — | — | 6 |
| Glioma | D005910 | EFO_0000520 | 3 | 4 | — | — | — | 5 | |
| Cholangiocarcinoma | D018281 | C22.1 | — | 5 | — | — | — | 5 |
Show 47 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Melanoma | D008545 | 3 | — | — | — | — | 3 | ||
| Thyroid neoplasms | D013964 | EFO_0003841 | 2 | — | — | — | — | 2 | |
| Healthy volunteers/patients | — | 2 | — | — | — | — | 2 | ||
| Kidney neoplasms | D007680 | EFO_0003865 | C64 | 1 | — | — | — | — | 1 |
| Digestive system neoplasms | D004067 | 1 | — | — | — | — | 1 | ||
| Respiratory tract neoplasms | D012142 | EFO_0003853 | D14 | 1 | — | — | — | — | 1 |
| Castleman disease | D005871 | EFO_1001332 | D47.Z2 | 1 | — | — | — | — | 1 |
| Soft tissue neoplasms | D012983 | 1 | — | — | — | — | 1 | ||
| Neurofibromatosis 2 | D016518 | Q85.02 | 1 | — | — | — | — | 1 | |
| Erdheim-chester disease | D031249 | 1 | — | — | — | — | 1 |
Show 6 more
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | CETUXIMAB |
| INN | cetuximab |
| Description | Immunoglobulin G 1 (human-mouse monoclonal C225 yt-chain anti-human epidermal growth factor receptor), disulfide with human-mouse monoclonal C225 x-chain, dimer |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 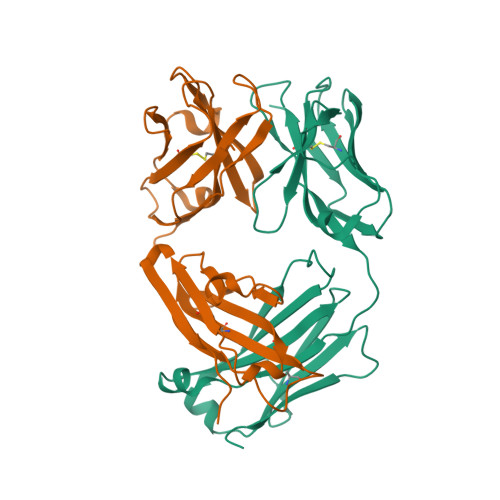 |
| Structure (InChI/SMILES or Protein Sequence) | >5GZ0:A,C|FM329 light chain
DIQMTQSPVILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGSPRLLIKYASESISGIPSRFSGSGSGTDFTLSINSVES
EDIADYYCQQNNNWPTTFGAGTKLELKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQ
ESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGAEC
>5GZ0:B,D|FM329 heavy chain
QVQLKQSGPGLVQPGGSLSITCTVSGFSLTNYGVHWVRQSPGKGLEWLGVIWSGGNTDYNTPFTSRLSINKDNSKSQVFF
KMNSLQSNDTAIYYCARALTYYDYEFAYWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSW
NSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKRVEPKSCDK |
Identifiers
| PDB | 1YY8, 1YY9, 4GW5, 4KRO, 4KRP, 5ESQ, 5ETU, 5EUK, 5F88, 5FF6, 5GZ0, 5HPM, 5HYQ, 5I2I, 5I76, 5ICX, 5ICY, 5ICZ, 5ID0, 5ID1, 5IOP, 5IR1, 5ITF, 5IV2, 5IVZ, 5T1K, 5T1L, 5T1M, 5TH2, 6ARP, 6ARU, 6AU5, 6AXP, 6AYN, 6AZK, 6AZL, 6B3S |
| CAS-ID | 205923-56-4 |
| RxCUI | 318341 |
| ChEMBL ID | CHEMBL1201577 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00002 |
| UNII ID | PQX0D8J21J (ChemIDplus, GSRS) |
Target
Agency Approved
EGFR
EGFR
Organism
Homo sapiens
Gene name
EGFR
Gene synonyms
ERBB, ERBB1, HER1
NCBI Gene ID
Protein name
epidermal growth factor receptor
Protein synonyms
avian erythroblastic leukemia viral (v-erb-b) oncogene homolog, cell growth inhibiting protein 40, cell proliferation-inducing protein 61, EGFR vIII, epidermal growth factor receptor tyrosine kinase domain, erb-b2 receptor tyrosine kinase 1, Proto-oncogene c-ErbB-1, Receptor tyrosine-protein kinase erbB-1
Uniprot ID
Mouse ortholog
Egfr (13649)
epidermal growth factor receptor (Q01279)
Alternate
No data
Variants
Clinical Variant
No data
Financial
Erbitux - Eli Lilly
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Erbitux - Bristol Myers Squibb
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 38,526 documents
View more details
Safety
Black-box Warning
Black-box warning for: Erbitux
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
482 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
