Bimagrumab
Bimagrumab is an antibody pharmaceutical. It is currently being investigated in clinical studies. The pharmaceutical is active against activin receptor type-2B. In addition, it is known to target activin receptor type-2A.
Download report
Favorite
Commercial
Therapeutic Areas
No data
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
New Drug Application (NDA)
New Drug Application (NDA)
Abbreviated New Drug Application (ANDA)
Abbreviated New Drug Application (ANDA)
No data
Labels
FDA
EMA
No data
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
ATC Codes
No data
HCPCS
No data
Clinical
Clinical Trials
13 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
No data
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Inclusion body myositis | D018979 | EFO_0007323 | G72.41 | — | 3 | 3 | — | — | 4 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Sarcopenia | D055948 | EFO_1000653 | M62.84 | — | 2 | — | — | — | 2 |
| Type 2 diabetes mellitus | D003924 | EFO_0001360 | E11 | — | 1 | — | — | — | 1 |
| Atrophy | D001284 | — | 1 | — | — | — | 1 | ||
| Artificial respiration | D012121 | — | 1 | — | — | — | 1 | ||
| Chronic obstructive pulmonary disease | D029424 | EFO_0000341 | J44.9 | — | 1 | — | — | — | 1 |
| Cachexia | D002100 | HP_0004326 | R64 | — | 1 | — | — | — | 1 |
| Obesity | D009765 | EFO_0001073 | E66.9 | — | 1 | — | — | — | 1 |
| Overweight | D050177 | E66.3 | — | 1 | — | — | — | 1 |
Indications Phases 1
No data
Indications Without Phase
No data
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | BIMAGRUMAB |
| INN | bimagrumab |
| Description | Bimagrumab (human mab) |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 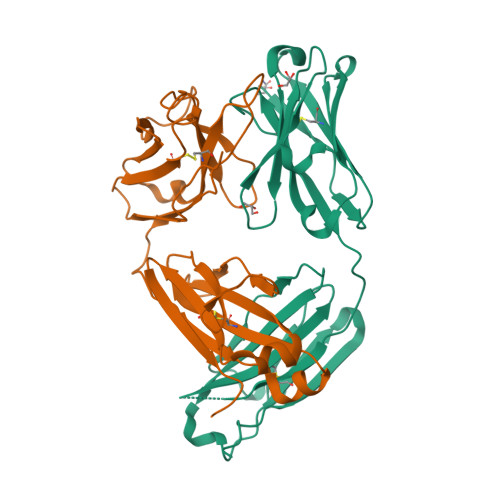 |
| Structure (InChI/SMILES or Protein Sequence) | >5NHW:H|anti-human ActRII Bimagrumab Fab heavy-chain
(PCA)VQLVQSGAEVKKPGASVKVSCKASGYTFTSSYINWVRQAPGQGLEWMGTINPVSGSTSYAQKFQGRVTMTRDTSI
STAYMELSRLRSDDTAVYYCARGGWFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSW
NSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSEFDYKDDDDKGAPHHHHHH
>5NHW:L|anti-human ActRII Bimagrumab Fab light-chain
QSALTQPASVSGSPGQSITISCTGTSSDVGSYNYVNWYQQHPGKAPKLMIYGVSKRPSGVSNRFSGSKSGNTASLTISGL
QAEDEADYYCGTFAGGSYYGVFGGGTKLTVLGQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTVAWKADSSPV
KAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQVTHEGSTVEKTVAPTEA |
Identifiers
| PDB | 5NGV, 5NH3, 5NHR, 5NHW |
| CAS-ID | 1356922-05-8 |
| RxCUI | — |
| ChEMBL ID | CHEMBL3137353 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB12584 |
| UNII ID | N15SW1DIV8 (ChemIDplus, GSRS) |
Target
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 293 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
5 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
