Avastin(bevacizumab)
Alymsys, Avastin, Mvasi, Vegzelma, Zirabev (bevacizumab) is an antibody pharmaceutical. Bevacizumab was first approved as Lextemy on . It is used to treat colorectal neoplasms, fallopian tube neoplasms, glioblastoma, kidney neoplasms, and non-small-cell lung carcinoma amongst others in the USA. It has been approved in Europe to treat breast neoplasms, colorectal neoplasms, fallopian tube neoplasms, non-small-cell lung carcinoma, and ovarian neoplasms amongst others. The pharmaceutical is active against vascular endothelial growth factor A. In addition, it is known to target Vascular endothelial growth factor A.
Download report
Favorite
Top 200 Pharmaceuticals by Retail Sales
Commercial
Trade Name
FDA
EMA
Alymsys, Avastin, Mvasi, Vegzelma, Zirabev
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Bevacizumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Avastin | bevacizumab | Genentech | N-125085 RX | 2004-02-26 | 2 products |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| colorectal neoplasms | — | D015179 | — |
| fallopian tube neoplasms | — | D005185 | — |
| glioblastoma | EFO_0000515 | D005909 | — |
| kidney neoplasms | EFO_0003865 | D007680 | C64 |
| non-small-cell lung carcinoma | — | D002289 | — |
| ovarian neoplasms | EFO_0003893 | D010051 | C56 |
| peritoneal neoplasms | — | D010534 | — |
| uterine cervical neoplasms | — | D002583 | — |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
bevacizumab, Avastin, Genentech, Inc. | |||
| 2027-05-29 | Orphan excl. | ||
Patent Expiration
No data
ATC Codes
L: Antineoplastic and immunomodulating agents
— L01: Antineoplastic agents
— L01F: Monoclonal antibodies and antibody drug conjugates
— L01FG: Vegf/vegfr (vascular endothelial growth factor) inhibitors
— L01FG01: Bevacizumab
S: Sensory organ drugs
— S01: Ophthalmologicals
— S01L: Ocular vascular disorder agents
— S01LA: Antineovascularisation agents
— S01LA08: Bevacizumab
Clinical
Clinical Trials
2264 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Colorectal neoplasms | D015179 | 60 | 224 | 82 | 7 | 34 | 372 | ||
| Non-small-cell lung carcinoma | D002289 | 35 | 127 | 48 | 2 | 13 | 213 | ||
| Breast neoplasms | D001943 | EFO_0003869 | C50 | 21 | 123 | 30 | 5 | 14 | 186 |
| Neoplasms | D009369 | C80 | 85 | 40 | 6 | 3 | 7 | 134 | |
| Ovarian neoplasms | D010051 | EFO_0003893 | C56 | 27 | 78 | 25 | 2 | 4 | 128 |
| Liver neoplasms | D008113 | EFO_1001513 | C22.0 | 26 | 54 | 9 | 2 | 8 | 88 |
| Macular degeneration | D008268 | EFO_0001365 | H35.30 | 10 | 27 | 23 | 5 | 18 | 75 |
| Macular edema | D008269 | 3 | 24 | 28 | 15 | 15 | 75 | ||
| Fallopian tube neoplasms | D005185 | 18 | 41 | 15 | 1 | 2 | 72 | ||
| Stomach neoplasms | D013274 | EFO_0003897 | C16 | 8 | 21 | 1 | 1 | — | 28 |
Show 22 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Glioblastoma | D005909 | EFO_0000515 | 44 | 95 | 11 | — | 7 | 137 | |
| Renal cell carcinoma | D002292 | 25 | 45 | 9 | — | 11 | 78 | ||
| Lung neoplasms | D008175 | C34.90 | 13 | 45 | 6 | — | 6 | 64 | |
| Rectal neoplasms | D012004 | 17 | 36 | 7 | — | 3 | 56 | ||
| Pancreatic neoplasms | D010190 | EFO_0003860 | C25 | 23 | 36 | 3 | — | 1 | 50 |
| Glioma | D005910 | EFO_0000520 | 14 | 28 | 1 | — | 6 | 43 | |
| Brain neoplasms | D001932 | EFO_0003833 | C71 | 12 | 28 | 1 | — | 5 | 41 |
| Colonic neoplasms | D003110 | C18 | 10 | 21 | 7 | — | 5 | 37 | |
| Gliosarcoma | D018316 | 7 | 29 | 1 | — | — | 33 | ||
| Uterine cervical neoplasms | D002583 | 5 | 18 | 6 | — | 1 | 29 |
Show 72 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Melanoma | D008545 | 9 | 29 | — | — | 2 | 35 | ||
| Healthy volunteers/patients | — | 21 | 1 | — | — | — | 22 | ||
| Sarcoma | D012509 | 8 | 12 | — | — | — | 19 | ||
| Esophageal neoplasms | D004938 | C15 | 4 | 14 | — | — | — | 18 | |
| Endometrial neoplasms | D016889 | EFO_0004230 | 2 | 14 | — | — | — | 15 | |
| Triple negative breast neoplasms | D064726 | 4 | 13 | — | — | — | 14 | ||
| Head and neck neoplasms | D006258 | 4 | 8 | — | — | — | 12 | ||
| Oligodendroglioma | D009837 | EFO_0000631 | 5 | 9 | — | — | — | 11 | |
| Central nervous system neoplasms | D016543 | 3 | 9 | — | — | — | 11 | ||
| Cholangiocarcinoma | D018281 | C22.1 | 3 | 7 | — | — | — | 10 |
Show 135 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cystadenocarcinoma | D003536 | 4 | — | — | — | — | 4 | ||
| Hematologic neoplasms | D019337 | 2 | — | — | — | — | 2 | ||
| Precursor cell lymphoblastic leukemia-lymphoma | D054198 | C91.0 | 2 | — | — | — | — | 2 | |
| Myelomonocytic leukemia chronic | D015477 | C93.1 | 2 | — | — | — | — | 2 | |
| Chondrosarcoma | D002813 | 2 | — | — | — | — | 2 | ||
| Pharmacokinetics | D010599 | 2 | — | — | — | — | 2 | ||
| Pharyngeal neoplasms | D010610 | C14.0 | 2 | — | — | — | — | 2 | |
| Hemangioblastoma | D018325 | 1 | — | — | — | 1 | 2 | ||
| Von hippel-lindau disease | D006623 | Q85.83 | 1 | — | — | — | 1 | 2 | |
| Lymphedema | D008209 | Q82.0 | 2 | — | — | — | — | 2 |
Show 35 more
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Vitrectomy | D014821 | — | — | — | — | 2 | 2 | ||
| Diabetes complications | D048909 | — | — | — | — | 2 | 2 | ||
| Hypertension | D006973 | EFO_0000537 | I10 | — | — | — | — | 2 | 2 |
| Wound infection | D014946 | — | — | — | — | 1 | 1 | ||
| Nerve tissue neoplasms | D009380 | — | — | — | — | 1 | 1 | ||
| Retinal drusen | D015593 | EFO_1001155 | — | — | — | — | 1 | 1 | |
| Intraocular pressure | D007429 | — | — | — | — | 1 | 1 | ||
| Molecular targeted therapy | D058990 | — | — | — | — | 1 | 1 | ||
| Myopia | D009216 | H52.1 | — | — | — | — | 1 | 1 | |
| Hereditary eye diseases | D015785 | — | — | — | — | 1 | 1 |
Show 8 more
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | BEVACIZUMAB |
| INN | bevacizumab |
| Description | Bevacizumab, sold under the brand name Avastin, is a medication used to treat a number of types of cancers and a specific eye disease. For cancer it is given by slow injection into a vein and used for colon cancer, lung cancer, glioblastoma, and renal-cell carcinoma. For age-related macular degeneration it is given by injection into the eye.
|
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 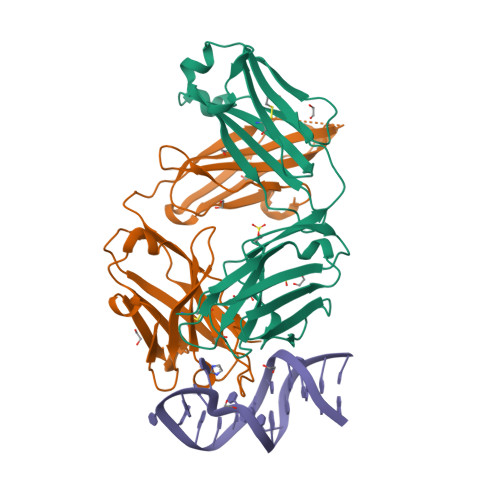 |
| Structure (InChI/SMILES or Protein Sequence) | >7V5N:A,E|bevacizumab fab light chain
DIQMTQSPSSLSASVGDRVTITCSASQDISNYLNWYQQKPGKAPKVLIYFTSSLHSGVPSRFSGSGSGTDFTLTISSLQP
EDFATYYCQQYSTVPWTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQ
ESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
>7V5N:B,F|bevacizumab fab heavy chain
EVQLVESGGGLVQPGGSLRLSCAASGYTFTNYGMNWVRQAPGKGLEWVGWINTYTGEPTYAADFKRRFTFSLDTSKSTAY
LQMNSLRAEDTAVYYCAKYPHYYGSSHWYFDVWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPV
TVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHT |
Identifiers
| PDB | 6BFT, 7V5N |
| CAS-ID | 216974-75-3 |
| RxCUI | 253337 |
| ChEMBL ID | CHEMBL1201583 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00112 |
| UNII ID | 2S9ZZM9Q9V (ChemIDplus, GSRS) |
Target
Agency Approved
VEGFA
VEGFA
Organism
Homo sapiens
Gene name
VEGFA
Gene synonyms
VEGF
NCBI Gene ID
Protein name
vascular endothelial growth factor A
Protein synonyms
vascular endothelial growth factor A121, vascular endothelial growth factor A165, Vascular permeability factor, VPF
Uniprot ID
Mouse ortholog
Vegfa (22339)
vascular endothelial growth factor A (Q00731)
Alternate
VEGFA
VEGFA
Organism
Homo sapiens
Gene name
VEGFA
Gene synonyms
NCBI Gene ID
—
Protein name
Vascular endothelial growth factor A
Protein synonyms
Vascular permeability factor
Uniprot ID
Mouse ortholog
—
—
Variants
Clinical Variant
No data
Financial
Aybintio - Organon
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Zirabev - Pfizer
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Avastin - Roche
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 74,805 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions
0 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
