Atoltivimab
Inmazeb (atoltivimab) is an antibody pharmaceutical. Atoltivimab was first approved as Inmazeb on 2020-10-14.
Download report
Favorite
FDA Novel Drug Approvals 2020
Commercial
Therapeutic Areas
No data
Trade Name
FDA
EMA
Combinations
Inmazeb
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Atoltivimab
+
Maftivimab
+
Odesivimab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Inmazeb | atoltivimab, maftivimab, and odesivimab-ebgn | Regeneron Pharmaceuticals | N-761169 RX | 2020-10-14 | 2 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| inmazeb | Biologic Licensing Application | 2020-10-14 |
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
atoltivimab / maftivimab / odesivimab, Inmazeb, Regeneron Pharmaceuticals, Inc. | |||
| 2027-10-14 | Orphan excl. | ||
Patent Expiration
No data
ATC Codes
No data
HCPCS
No data
Clinical
Clinical Trials
3 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
No data
Indications Phases 3
No data
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Ebola hemorrhagic fever | D019142 | EFO_0007243 | A98.4 | — | 1 | — | — | 1 | 2 |
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers/patients | — | 1 | — | — | — | — | 1 |
Indications Without Phase
No data
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | ATOLTIVIMAB |
| INN | atoltivimab |
| Description | Atoltivimab is a Zaire ebolavirus glycoprotein-directed human monoclonal antibody that is part of the fixed-dose combination atoltivimab/maftivimab/odesivimab that is used for the treatment of Zaire ebolavirus (Ebola virus).
|
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 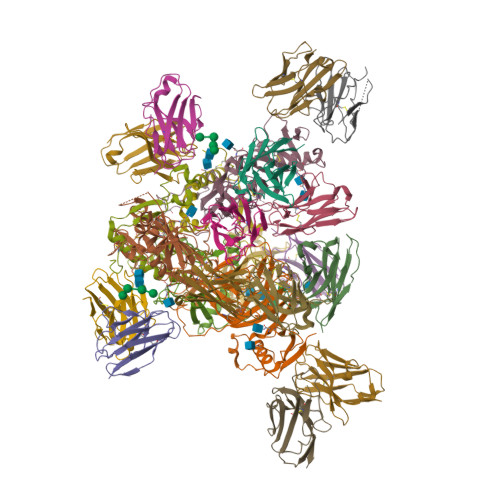 |
| Structure (InChI/SMILES or Protein Sequence) | >7TN9:A,C,E|REGN3471 heavy chain
EVQLVESGGGLVQPGGSLRLSCAASGFTFSSYDMHWVRQATGKGLEWVSAIGTAGDTYYPGSVKGRFTISRENAKNSLYL
QMNSLRAGDTAVYYCARTWFGELYFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWN
SGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVE
>7TN9:F,B,D|REGN3471 light chain
DIVMTQSPDSLAVSLGERATINCKSSQSVLYSSNNKNYLAWYQQKPGQPPKLLIYWASTRESGVPDRFSGSGSGTEFTLT
ITSLQAEDVAVYYCQQYYSSPLTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNAL
QSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
>7TN9:G,I|REGN3470 heavy chain
QVQLVESGGGVVQPGRSLRLSCAASGFTFNNYGMHWVRQAPGMGLEWVAVIWHDGSDKYYADSVKGRFTISRDNSKNTLY
LQMNSLRAEDTAVYYCARNWNLFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSG
ALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVE
>7TN9:H,J|REGN3470 light chain
DIQMTQSPSSLSASVGDRITITCRASQSISTYLHWYQQKPGKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQP
EDFATYYCQQSFSTPPINFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNS
QESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
>7TN9:Q,M,O|REGN3479 heavy chain
EVQLVESGGGLVQPGGSLRLSCAASGFTSSSYAMNWVRQAPGKGLEWVSTISGMGGSTYYADSVKGRFTISRDNSKNTLY
LQMNSLRAEDTAVYYCAKRGYPHSFDIWGQGTMVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWN
SGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVE
>7TN9:R,N,P|REGN3479 light chain
DIQMTQSPSSLSASVGDRVTITCRASQSISSFLNWYQQKPGKAPKLLIYAASSLQSGVPSRFSGSGSGTDFTLTISSLQP
EDFATYYCQQSYSTLTFGQGTRLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQE
SVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
>7TN9:T,S,U|Envelope glycoprotein
RSIPLGVIHNSTLQVSDVDKLVCRDKLSSTNQLRSVGLNLEGNGVATDVPSATKRWGFRSGVPPKVVNYEAGEWAENCYN
LEIKKPDGSECLPAAPDGIRGFPRCRYVHKVSGTGPCAGDFAFHKEGAFFLYDRLASTVIYRGTTFAEGVVAFLILPQAK
KDFFSSHPLREPVNATEDPSSGYYSTTIRYQATGFGTNETEYLFEVDNLTYVQLESRFTPQFLLQLNETIYTSGKRSNTT
GKLIWKVNPEIDTTIGEWAFWETKKNLTRKIRSEELSFTVVNTHHQDTGEESASSGKLGLITNTIAGVAGLITGGRRTRR
E
>7TN9:W,V,X|GP2
AIVNAQPKCNPNLHYWTTQDEGAAIGLAWIPYFGPAAEGIYTEGLMHNQDGLICGLRQLANETTQALQLFLRATTELRTF
SILNRKAIDFLLQRWGGTCHILGPDCCIEPHDWTKNITDKIDQIIHDFVDKTLPDDDDDK |
Identifiers
| PDB | 7TN9 |
| CAS-ID | 2135632-29-8 |
| RxCUI | — |
| ChEMBL ID | CHEMBL4298183 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB15898 |
| UNII ID | FJZ07Q63VY (ChemIDplus, GSRS) |
Target
Agency Approved
No data
Alternate
No data
Variants
Clinical Variant
No data
Financial
Inmazeb - Regeneron Pharmaceuticals
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 114 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
19,131 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
