Alteplase
Activase (alteplase) is an enzyme pharmaceutical. Alteplase was first approved as Activase on 1987-11-13. It is used to treat arterial occlusive diseases, intracranial embolism and thrombosis, myocardial infarction, pulmonary embolism, and stroke in the USA.
Download report
Favorite
Commercial
Trade Name
FDA
EMA
Activase, Cathflo activase
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| cathflo activase | Biologic Licensing Application | 2020-10-13 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| arterial occlusive diseases | EFO_0009085 | D001157 | — |
| intracranial embolism and thrombosis | — | D002542 | — |
| myocardial infarction | EFO_0000612 | D009203 | I21 |
| pulmonary embolism | EFO_0003827 | D011655 | I26 |
| stroke | EFO_0000712 | D020521 | I63.9 |
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| G8601 | Iv alteplase not initiated within three hours (<= 180 minutes) of time last known well for reasons documented by clinician (e.g. patient enrolled in clinical trial for stroke, patient admitted for elective carotid intervention, patient received tenecteplase (tnk)) |
| J2997 | Injection, alteplase recombinant, 1 mg |
Clinical
Clinical Trials
239 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | D020521 | EFO_0000712 | I63.9 | 3 | 17 | 17 | 3 | 22 | 59 |
| Ischemic stroke | D000083242 | 2 | 17 | 18 | 4 | 16 | 52 | ||
| Pulmonary embolism | D011655 | EFO_0003827 | I26 | 1 | 3 | 8 | 7 | 1 | 20 |
| Thrombosis | D013927 | 2 | — | 2 | 2 | 2 | 8 | ||
| Pleural effusion | D010996 | J90 | — | 1 | — | 2 | 2 | 5 | |
| Venous thrombosis | D020246 | HP_0004936 | I82.40 | — | 1 | 2 | 1 | — | 4 |
| Empyema | D004653 | EFO_0003097 | — | 1 | 2 | 1 | 1 | 4 | |
| Right ventricular dysfunction | D018497 | — | — | 1 | 2 | — | 3 | ||
| St elevation myocardial infarction | D000072657 | — | — | 1 | 2 | — | 3 | ||
| Pleural diseases | D010995 | — | — | — | 3 | — | 3 |
Show 9 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Myocardial infarction | D009203 | EFO_0000612 | I21 | — | 1 | 7 | — | 1 | 9 |
| Cerebral intraventricular hemorrhage | D000074042 | — | 2 | 1 | — | 2 | 5 | ||
| Respiratory distress syndrome | D012128 | EFO_1000637 | J80 | — | 2 | 2 | — | — | 3 |
| Cardiovascular diseases | D002318 | EFO_0000319 | I98 | — | — | 3 | — | — | 3 |
| Coronary disease | D003327 | — | — | 3 | — | — | 3 | ||
| Myocardial ischemia | D017202 | EFO_1001375 | I20-I25 | — | — | 3 | — | — | 3 |
| Heart diseases | D006331 | EFO_0003777 | I51.9 | — | — | 3 | — | — | 3 |
| Subarachnoid hemorrhage | D013345 | EFO_0000713 | I60 | 1 | 2 | 1 | — | — | 3 |
| Cerebral hemorrhage | D002543 | — | 2 | 1 | — | — | 3 | ||
| Abdominal abscess | D018784 | EFO_1001753 | — | 1 | 1 | — | 1 | 3 |
Show 19 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Covid-19 | D000086382 | U07.1 | — | 1 | — | — | 2 | 3 | |
| Respiratory insufficiency | D012131 | HP_0002093 | J96.9 | — | 2 | — | — | — | 2 |
| Healthy volunteers/patients | — | 1 | 1 | — | — | — | 2 | ||
| Hypertensive intracranial hemorrhage | D020299 | — | 1 | — | — | 1 | 2 | ||
| Neoplasms | D009369 | C80 | 1 | 2 | — | — | — | 2 | |
| Upper extremity deep vein thrombosis | D056824 | — | 1 | — | — | — | 1 | ||
| Brain infarction | D020520 | EFO_0004277 | I63 | — | 1 | — | — | — | 1 |
| Lung neoplasms | D008175 | C34.90 | — | 1 | — | — | — | 1 | |
| Central venous catheterization | D002405 | — | 1 | — | — | — | 1 | ||
| Nervous system diseases | D009422 | G00-G99 | — | 1 | — | — | — | 1 |
Show 7 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Retinal vein occlusion | D012170 | EFO_1001157 | H34.81 | 2 | — | — | — | — | 2 |
| Malignant pleural effusion | D016066 | J91.0 | 1 | — | — | — | — | 1 | |
| Embolism | D004617 | 1 | — | — | — | — | 1 | ||
| Thrombophlebitis | D013924 | HP_0004418 | 1 | — | — | — | — | 1 | |
| Glaucoma | D005901 | EFO_0000516 | H40 | 1 | — | — | — | — | 1 |
| Adjuvant chemotherapy | D017024 | 1 | — | — | — | — | 1 | ||
| Peritoneal dialysis | D010530 | 1 | — | — | — | — | 1 | ||
| Continuous ambulatory peritoneal dialysis | D010531 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Macular edema | D008269 | — | — | — | — | 1 | 1 | ||
| Sars-cov-2 | D000086402 | — | — | — | — | 1 | 1 | ||
| Liver cirrhosis | D008103 | EFO_0001422 | K74.0 | — | — | — | — | 1 | 1 |
| Coagulation protein disorders | D020147 | — | — | — | — | 1 | 1 | ||
| Depression | D003863 | F33.9 | — | — | — | — | 1 | 1 | |
| Anxiety disorders | D001008 | EFO_0006788 | F41.1 | — | — | — | — | 1 | 1 |
| Mortality | D009026 | EFO_0004352 | — | — | — | — | 1 | 1 | |
| Thrombolytic therapy | D015912 | — | — | — | — | 1 | 1 | ||
| Basal ganglia hemorrhage | D020145 | — | — | — | — | 1 | 1 | ||
| Brain injuries | D001930 | S06.9 | — | — | — | — | 1 | 1 |
Show 10 more
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | ALTEPLASE |
| INN | alteplase |
| Description | Alteplase (t-PA) is a thrombolytic medication, used to treat acute ischemic stroke, acute ST-elevation myocardial infarction (a type of heart attack), pulmonary embolism associated with low blood pressure, and blocked central venous catheter. It is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.
|
| Classification | Enzyme |
| Drug class | enzymes: tissue-type plasminogen activators |
| Image (chem structure or protein) | 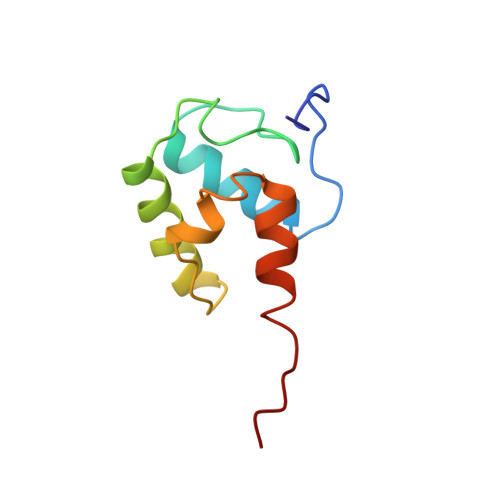 |
| Structure (InChI/SMILES or Protein Sequence) | — |
Target
Agency Approved
No data
Alternate
No data
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 12,359 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
53,940 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
