Tools for Clinical Trial Success Forecasting
The cost of clinical trials can be greater than 50% of the overall cost of new drug development.
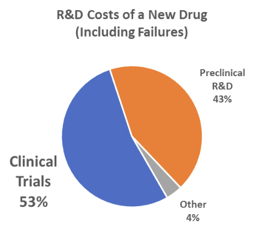 According to a report by the US Congressional Budget Office (CBO) released in April of 2021, the cost to develop a new drug—including capital costs and expenditures on drugs that fail to reach the market—has been estimated to range from less than $1 billion to more than $2 billion. The CBO indicates that drug companies report the cost of clinical trials can reach over $1 billion of the overall drug development cost.
According to a report by the US Congressional Budget Office (CBO) released in April of 2021, the cost to develop a new drug—including capital costs and expenditures on drugs that fail to reach the market—has been estimated to range from less than $1 billion to more than $2 billion. The CBO indicates that drug companies report the cost of clinical trials can reach over $1 billion of the overall drug development cost.
Because of the high financial impact of trials, financial investors and pharmaceutical leaders monitor the progress of clinical trials carefully. Successful completion of clinical trials will likely lead to FDA approval and a corresponding escalation in market value of the sponsoring company. On the other hand, failure in completing a clinical trial can lead to a reduction in a company’s market value.
PharmaKB enables detailed analysis of current and past clinical trials.
PharmaKB collects detailed information on active clinical trials daily. This includes all important dates, related milestones, and trials descriptions. PharmaKB employs machine learning (ML) to recognize and categorize disease indications being tested in all trials. Disease indications are mapped to standard MeSH (Medical Subject Heading) terminology used by the FDA in reporting approval status.
PharmaKB’s normalized trials data allows PharmaKB to present all approved drugs and trial-stage drugs that are related to any FDA recognized disease indication along with important time-based events.
PharmaKB trials data enables the calculation of the likelihood that a drug candidate will successfully advance from Phase 1 through Approval (see pop-up below).
The graphic (left) illustrates an example calculation of the likelihood of candidate drugs in clinical trials embedded within the PharmaKB disease browsing application.
As the user browses for current and future commercial pharmaceutical products related to a disease market, the user is given interactive feedback on past clinical trials success rates for drugs focused on treating that disease.
PharmaKB clinical trials data is available for download by subscribers.
Properly licensed subscribers to The Pharmaceutical KnowledgeBase may download files and documentation through the PharmaKB Market Intelligence data access page.
In order to develop and refine financial and other performance models for commercial drugs, analysts require regularly updated data on all key facets of drug performance ranging from financial to safety.
Collaborative Drug Discovery (CDD), Inc. addresses this need by offering a cost-effective solution for reviewing the commercial status of all US FDA approved drugs. The Pharmaceutical KnowledgeBase is available at (www.pharmakb.com).
PharmaKB provides up-to-date and detailed information about drugs, companies, and disease areas so that commercial organizations can gain a precise picture of where commercial opportunities lie based on impacting factors such as patent and exclusivity termination, competitive product introduction, detection of new safety concerns, and many others.
All content is available through the PharmaKB web application at (www.pharmakb.com) or via the PharmaKB API (details at www.pharmakb.com/pharmakb-api-documentation) and via GitHub .
For information on the PharmaKB content and/or subscription-based access, please contact us at: info@pharmakb.com
