PharmaKB Case Study to identify Combination Therapies in Clinical Trials for COVID-19
PharmaKB1 is a new data offering from Collaborative Drug Discovery. PharmaKB collects and collates a broad range of drug information, allowing users to fully explore the triad of company, drug, and disease. In this case study we explore how data from the COVID-19 drug set can be used to provide an overview of the drug landscape, generating insights into drug development strategies and drug safety profiles.
Using PharmaKB, we reviewed key information for drugs that have been investigated as therapies for COVID-19. The 10 most frequently evaluated drugs in clinical trials against COVID-19, excluding heparin, as of April 2021 were grouped into a select set from PharmaKB’s more expansive COVID-19 Potential Therapeutics drug set.
Of these, only 2 drugs were licensed for use in this disease. Remdesivir was licensed by both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA), while dexamethasone was licensed by the EMA.2-5
Drugs were divided by target or Anatomical Therapeutic Chemical Classification (ATCC) code into three classes:
- Drugs that act on viral proteins,
- drugs that act on the immune system, or
- other targets.
We also used the clinical trial information in PharmaKB to identify the drugs that had been investigated as combination therapy (excluding standard of care combinations).
Of the 10 drugs in the COVID-19 drug subset, the majority (7) target viral proteins and just under a third (3) target the immune system. We found that viral- and immune-targeting drugs were evaluated in combination with other key therapies at a similar frequency (range 4-8 and 5-8 therapy combinations, Figure 1).
In particular, tocilizumab and ritonavir had been studied in combination with 8 of the other frequently evaluated drugs. Combination therapies included the use of multiple viral-targeting drugs, possibly as a multi-faceted approach to reduce the viral load and resolve the infection.
Viral-targeting drugs were also frequently evaluated in combination with immune-targeting drugs, possibly to reduce the harmful effects of inflammation while providing treatment for the infection.
To assess how drug combination therapy might contribute to the adverse drug reactions (ADRs) experienced by patients, we used the safety information provide in PharmaKB (based on US FDA Adverse Event Reporting System) to evaluate the potential for cumulative toxicity.
We selected ADRs based on the following criteria:
- Occur at a frequency above an estimated threshold based on an average drug profile.
- Common (≥40%) among the drugs contained in the select COVID-19 drug set.
- Frequently experienced by patients (representing ≥5% of all ADRs reported for that drug)
Arthralgia, diarrhea, fatigue, headache, nausea, and rash met these criteria, suggesting that patients treated with combinations of these drugs may experience a higher incidence or greater severity of such adverse reactions compared with monotherapy regimens (Figure 2).
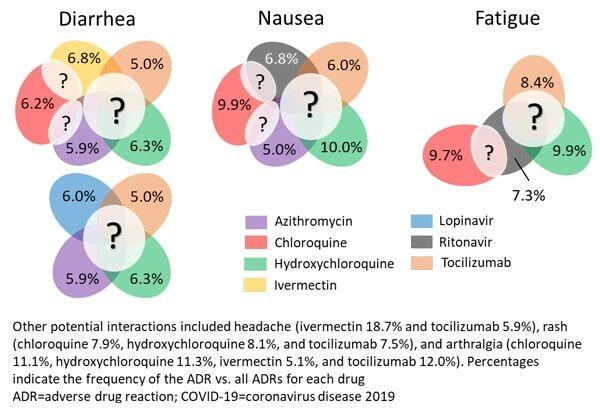
Figure 2. Frequent (≥5%) ADRs common (≥40%) between drugs under investigation as combination therapy against COVID-19
Therefore, clinical trials evaluating such combination therapies should carefully monitor for these events. If present, these ADRs would impact patient compliance with treatment schedules, drug concentrations, and the long-term health of patients and their quality of life.
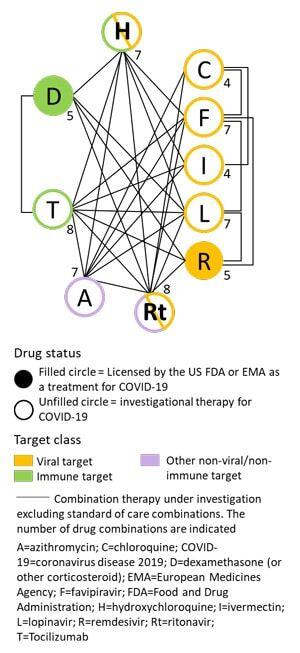
Figure 1. Status and target class of key drugs under investigation for efficacy against COVID-19 in clinical trials
Overall, this case study indicates how BioHarmony can be used to investigate trends in current drug development strategies, generating new hypotheses to inform future preclinical and clinical studies.
Bibliography and Resources
- www.pharmakb.com. Accessed April 21, 2021
- Gilead Sciences, Inc. Veklury® (remdesivir) for injection, for intravenous use. US Prescribing Information; 10/2020. accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf. Accessed April 2021.
- Gilead Sciences Ireland UC. Veklury® (remdesivir). EU Summary of Product Characteristics; 12/2020. https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_en.pdf. Accessed April 2021.
- European Medicines Agency. Outcome of Art 5(3) procedure – Product Information (for dexamethasone only-containing oral or IV medicinal products). https://www.ema.europa.eu/en/documents/other/dexamethasone-covid19-article-53-procedure-proposals-product-information_en.pdf. Accessed April 2021.
- European Medicines Agency. EMA endorses use of dexamethasone in COVID-19 patients on oxygen or mechanical ventilation; 2021. https://www.ema.europa.eu/en/news/ema-endorses-use-dexamethasone-covid-19-patients-oxygen-mechanical-ventilation. Accessed April 2021.
